
Our expertise
CISMaP Center of Excellence specializes in the field of Diagnostics and Medical Devices, including:
- Analyzing chemical properties and characteristics using Fourier Transform Infrared Spectroscopy (FTIR)
- Analyzing molecular components using FTIR-ATR mode and classifying components using OPUS database (ISO/IEC 17025:2017 accredited)
- Providing research tools for both public and private sectors, such as analyzing various cancer markers, synthesizing materials for materials science and dentistry, and applying technologies in the food industry
- Offering consultation on FTIR techniques, spectroscopy pilot studies, and result analysis
- Molecular genetics-based Thalassemia testing in neonates and postnatal cases
- Providing proficiency testing for medical laboratory practices (ISO/IEC 17043 accredited)
- Automated blood culture program
- Thalassemia diagnostic program
- Manufacturing Quality Control (QC) materials for laboratory use (ISO/IEC 17034 preparation stage)
- QC materials for clinical microbiology laboratory culturing
- QC materials for HbA1C analysis to indicate blood sugar control
- Researching biosensors and developing lateral flow strip prototypes
- Spraying services
- Result interpretation services using automated readers
- Handling large-scale data and AI analytics, particularly in health-related domains
- Providing an AI program for assessing body age and vital organs (Health AI program)
- Expertise in Sensor technology: Chemical sensors, Mass sensors
History
Center for Innovation and Standard for Medical Technology and Physical Therapy was established as a strategic unit under the Faculty of Medical Technology, Khon Kaen University. It was founded with support from the National Research Council of Thailand's D1 research grant in 2018. The project titled 'Standardization Center to Support the Research Network in Disease Diagnosis Technology Using Biological Infrared Spectroscopy and an International-Level Medical Laboratory Proficiency Testing Center' aims to fulfill the mission of the Faculty of Medical Technology in terms of translating research and innovation into practical applications. The center's goals include providing academic services in medical and health sciences, developing personnel expertise in health science, and enhancing the country's competitive edge by advancing research, development, and knowledge in medical and health sciences."
Rationale for Establishing
Faculty of Medical Technology at Khon Kaen University are centered around its core competency in research to address significant and common health problems in the region. These issues have an impact on the quality of life and can be translated into healthcare services. The faculty recognizes the importance of contributing to driving Thailand towards the Thailand 4.0 era in the fields of public health, healthcare, and medical technology.
To achieve this, the faculty has established a standardized laboratory to support a research network in diagnostic technology. The focus is on research in bio-spectroscopy platform, particularly for disease diagnosis. The development of measurement standards is emphasized, benchmarked against reference methods. The goal is to establish a standardized research laboratory for the bio-spectroscopy platform, serving as a diagnostic technology for pre-disease surveillance, early disease screening, and continuous treatment monitoring. Accurate and rapid disease progression assessment will enhance the quality of life for the population and reduce the consequences of chronic illnesses.
Moreover, this technological laboratory platform can serve as a reference and support the application of research benefits to researchers in the region. Collaborating with experts from both domestic and international sources, including institutions such as the Synchrotron Light Research Institute, a private sector organization, and Monash University in Australia, the laboratory aims to consult and develop the mentioned technologies.
Furthermore, in line with the Faculty of Medical Technology's capacity expansion plan, it aims to encompass the certification and quality control of medical laboratory diagnostic capabilities. As part of this effort, an international-level medical laboratory proficiency testing center has been established. This center follows a quality management process that encompasses organizational integration, strategic planning, emphasis on leadership and stakeholder involvement, data analysis and management, personnel focus, process management, in conjunction with compliance to standards and ISO 17043 (Proficiency testing provider) requirements.
Objectives and scope
- The objectives and scope of operations of the center include providing analytical testing services for components using infrared spectroscopy under the ISO/IEC 17025:2017 standard.
- The center aims to serve as a proficiency testing center for medical laboratory operations. Currently, it has produced proficiency testing materials for blood culture-related microbiological processes (Hemoculture) under the ISO/IEC 17043:2010 standard.
- Additionally, the center offers analytical testing services using infrared spectroscopy for research, academic, and private sector purposes. This includes providing consultation on infrared spectroscopy techniques and data analysis.
Collaborations
The collaboration involves consulting with other organizations regarding infrared spectroscopy techniques.
- Partnerships include:
- Synchrotron Light Research Institute, a private sector organization.
- Monash University, Australia.
Collaboration in producing proficiency testing materials for blood culture-related microbiological processes (Hemoculture) for the private sector, including firms like Fermier Co., Ltd. and Innomed (Thailand) Co., Ltd.
Products and Services with International Standards
- Under ISO/IEC 17025:2017, the laboratory provides analytical testing services using the infrared spectroscopy technique. Analysis items: Analysis of components using infrared spectroscopy. Test material: Kidney stone. Status: In the certification process by the Department of Science Service.
- Under ISO/IEC 17043:2010, the laboratory organizes proficiency testing programs for medical laboratory capabilities. Program: Automated blood culture proficiency testing program. Status: In the certification process by the Department of Science Service.
- Under ISO/IEC 17034:2016, certified reference material producer capabilities are being established. Status: In the system development phase.
- Additionally, the organization is undergoing certification from food and drug authorities for product manufacturing and distribution.
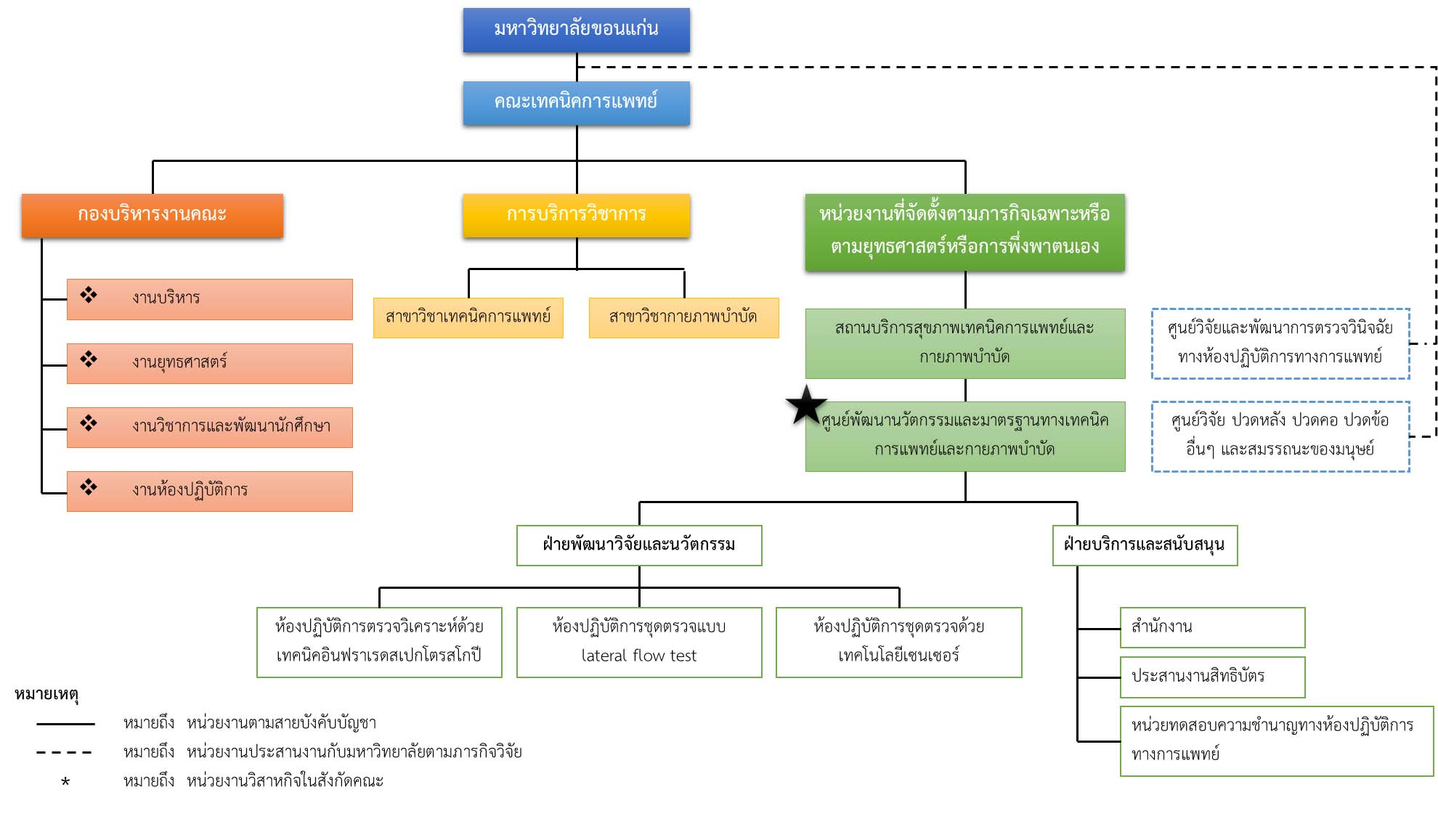
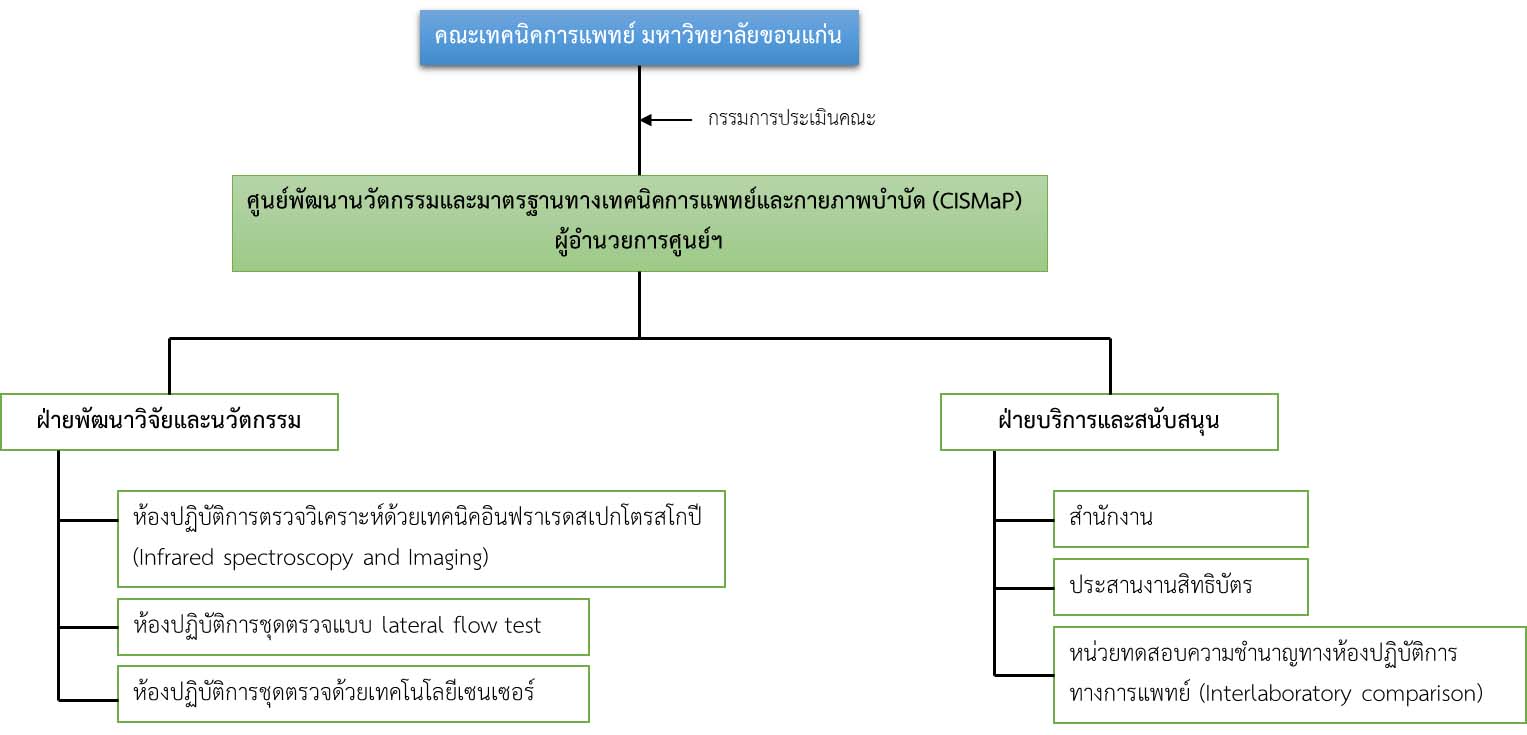
Organizational chart
Products and Innovation
Microbial thin film
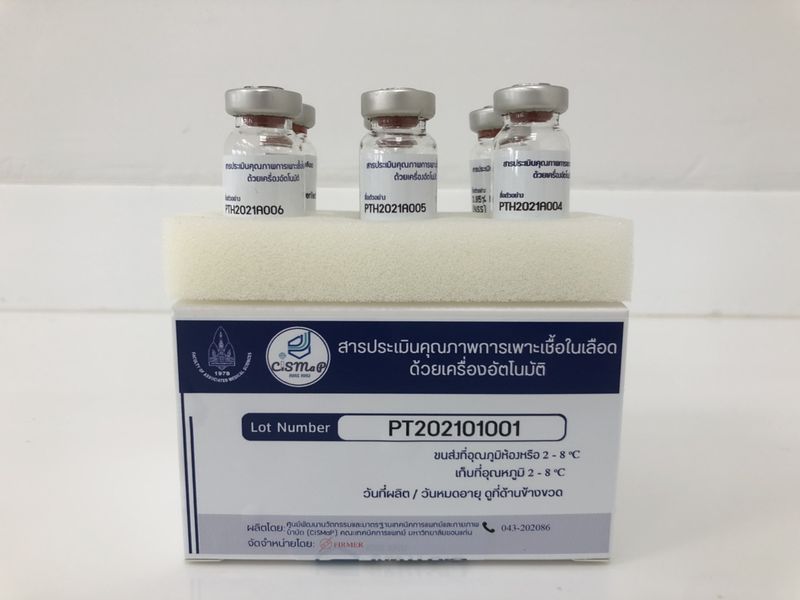
Description
This product preserves viable microorganisms in a thin film format, and can be used as a standard material for evaluating the staff competency and facilitating certified proficiency testing of hemoculture or bacterial culture system among clinical laboratories.
Highlights
The microbial film is highly stable when or still alive when stored at 4 ºC for a year. It is also stable during transport at room temperature temperatures (no more than 40ºC) for up to 2 weeks.
Related standards
ISO/IEC 17034:2016 (Reference materials)
ISO/IEC 17043:2010 (Proficiency test providers)
Service
Kidney stone composition analysis
-
Descriptive service
Analysis of stone composition using infrared spectroscopy (ATR mode). The identities of chemicals are determined using the OPUS database following the international standard of laboratory testing (ISO 17025:2017).
-
Related standards
ISO/IEC 17025:2017
-
Service form
-
Service guide describing request and report forms
-
Infrared spectroscopy analysis of kidney stones (firstly approved ISO17025 inThailand) including R&D of both government and private organizations.
Providing consultations and training programs related to this technique and the data analysis thereof.

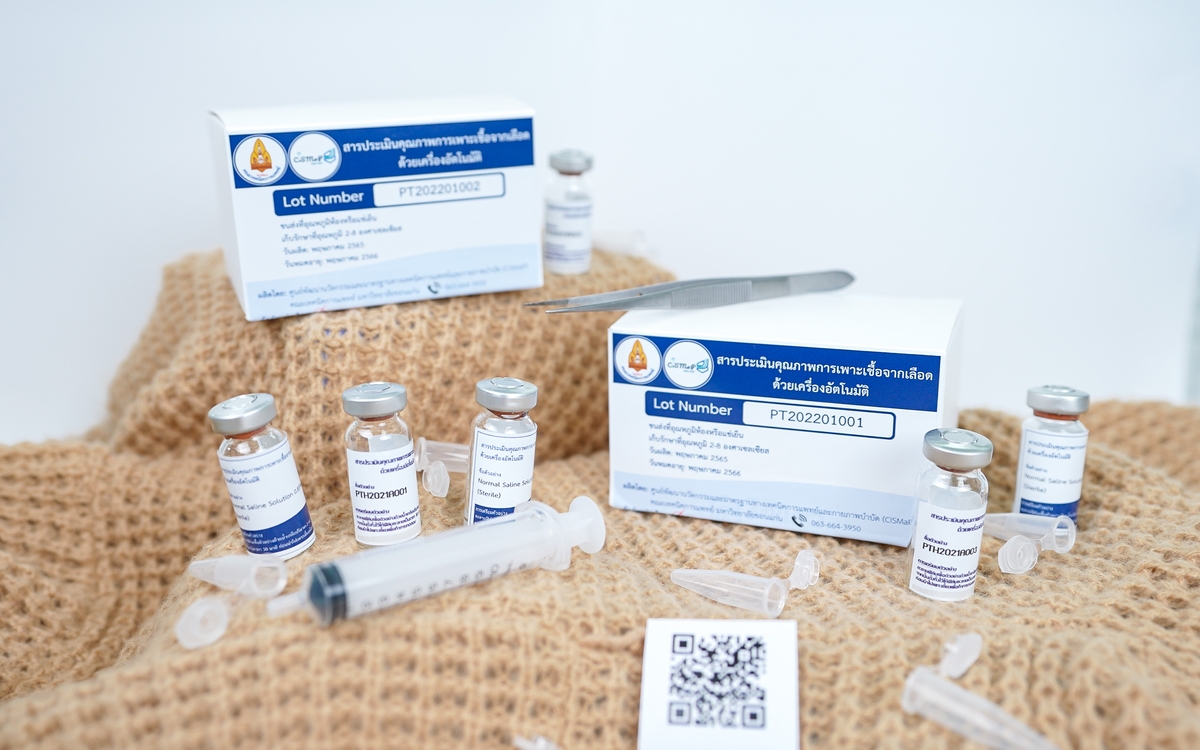
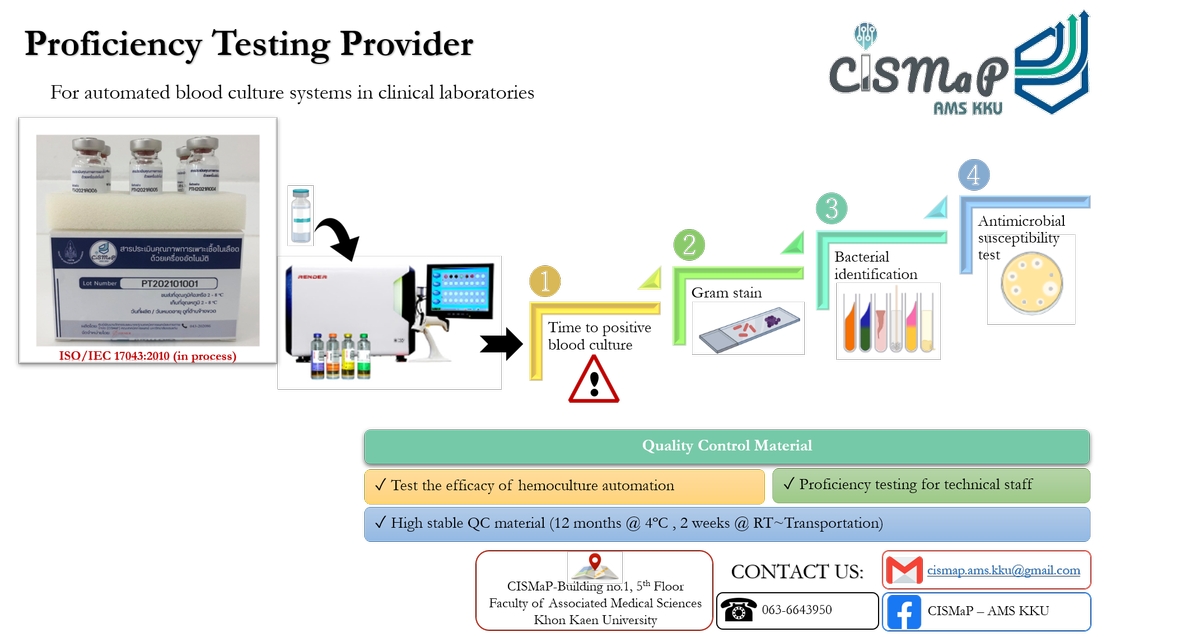
Proficiency testing for automated hemoculture systems in clinical laboratories
-
Descriptive service
QC materials are provided for the following parameters in hemoculture:
- Time to positivity of blood cultures
- Gram stain
- Bacterial identification
- Antimicrobial susceptibility test
-
Related standards
ISO/IEC 17043:2010
-
Service form
https://docs.google.com/forms/d/e/1FAIpQLSc70xSRtPaontqCUb2k909nlJ0nFnCqXa-Fsj0za-3_LhOudA/viewform
-
Service guide
-
Registration and data processing on Web based platform. Service under international standard ISO 17043
Health AI for Biological Age and Importance Organs
- The HealthAI program reports comprehensive body age relative to calendar age, as well as the estimated ages of vital organs including liver, kidney, heart, and pancreas
- The underlying algorithm was constructed using data collected from the Thai population. The prediction accuracy is 83.70% for females and 81.50% for males when compared with the standard method for body age assessment.
- Reports that are easy to understand and continuous records which serve as a valuable addition to conventional health check-up reports.

Our expertise
- Compositional analysis of stones using ATR-FTIR spectroscopy
- Consultations on FTIR spectroscopy, imaging microscopy, and data analysis for research in medicine and other disciplines
- Construction of prototypes for biosensors and lateral flow strips
- Consultations on the preparation of laboratories for international certifications including ISO 15189, ISO 17025, ISO 17043
Equipment and Facilities
- TIR Imaging Microscopes and Spectrometers (Models: Tensor II and Hyperion 3000). Also available for use in a temperature and humidity control room
- Agilent Technologies 4500 Series FTIR in a temperature and humidity control room
- Biological safety cabinet class II
- Design and development for rapid test using lateral flow strip
- Centrifuge
- Dehumidifier (model: OL70-585E)
- Cobas e411 autoanalyzer



Contact
CISMaP - AMS KKU https://www.facebook.com/CISMaPKKU/
cismap.ams.kku@gmail.com
+666 3664 3950, +66 4320 2399 ext. 50696
CISMaP, 5th floor
Faculty of Associated Medical Sciences, Building 1
Khon Kaen University, 123 Mittraphap Road, Tambon Nai Mueang, Amphoe Mueang Khon Kaen, Khon Kaen 40002

