
Our expertise
QDD offers a variety of services related to diagnostics and medical devices, including fabrication, development, and validation of lateral flow strip assays. Our main goal is to support researchers through across all stages of transforming their prototypes to high-quality commercial kits, by providing standardized facilities and comprehensive validation services.

History
The Qualified Diagnostic Development center (QDD) at Chulalongkorn University was initiated by Council of University Presidents of Thailand, which acknowledges the growing importance of establishing infrastructure for developing diagnostic platform technologies and promoting an innovation-based economy, in line with the Thailand 4.0 policy related to aging society. To this end, the council assigned seven public universities to submit proposals for the project entitled “GMP (Good Manufacturing Practices) laboratories for the development of rapid diagnostic kits.”
Rationale for Establishing
The primary function of QDD is to develop diagnostic test kits that meet international standards, and to serve as a method validation center that operates based on reliable research. In addition, QDD is responsible for helping transform diagnostic prototypes to commercial kits, as well as preparing laboratories to meet the international standard ISO 13485, under the scope “Design and Development and Production of Lateral Flow Immunochromatographic Strip Test” from SGS Thailand. The center received a certification from SGS, UKAS, United Kingdom on January 9, 2023. Furthermore, QDD has played a crucial role in coordinating, accelerating, and validating research projects with high potential from within and outside of Chulalongkorn University, in order to obtain prototypes that are certified for production under the international standard ISO 13485.
Objectives and scope
Established as a focal point for the development of diagnostic test kits in Thailand, the Qualified Diagnostic Development center (QDD) has operated with the following key missions:
- Provision of services related to the development of rapid diagnostic test kits, including upscaling, and prototype preparation, in order to promote their implementation in real-world settings and commercialization.
- Preparation of prototypes of rapid diagnostic tests in sufficient quantities for clinical or field trials, method validation, and transition to industrial-scale manufacturing.
Collaborations
- Collaborate with various research institutions within Chulalongkorn University, including Faculty of Science, Faculty of Medicine, Faculty of Veterinary Science, Faculty of Allied Health Science, The Institute of Biotechnology and Genetic Engineering, among others, as well as research institutions outside of the university.
- Facilitate the transfer of validated diagnostic technologies to relevant private organizations for manufacturing.
- Engage and coordinate with relevant governmental organizations, including the Department of Livestock Development, public hospitals, health centers, and others, to encourage the adoption and utilization of validated tests.
Products and Services with International Standards
TThe manufacturing facility of QDD has been registered as medical device manufacturing establishment with the Food and Drug Administration (FDA) of Thailand (registration number: กท.สผ.182/2563), in the categories of medical devices use in Veterinary, and Clinical laboratory.
It has also obtained certification in compliance with the ISO 13485:2016 standard, for 'Design and Development and Production of Lateral Flow Immunochromatographic Strip Test.'
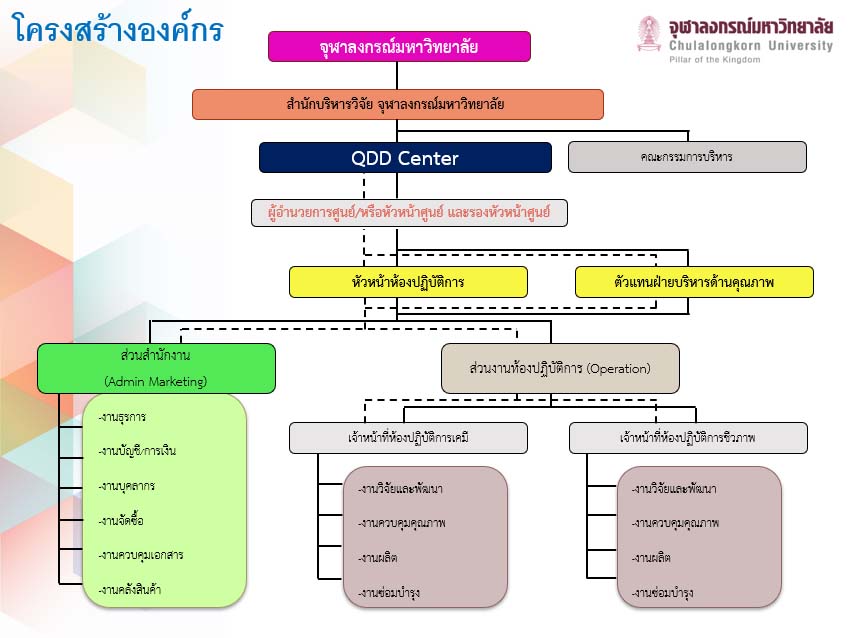
Organizational chart
Products and Innovation
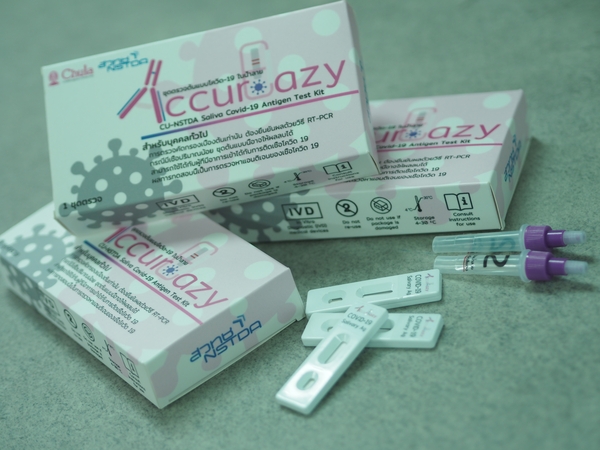
Antigen test kit for screening of COVID-19 in saliva
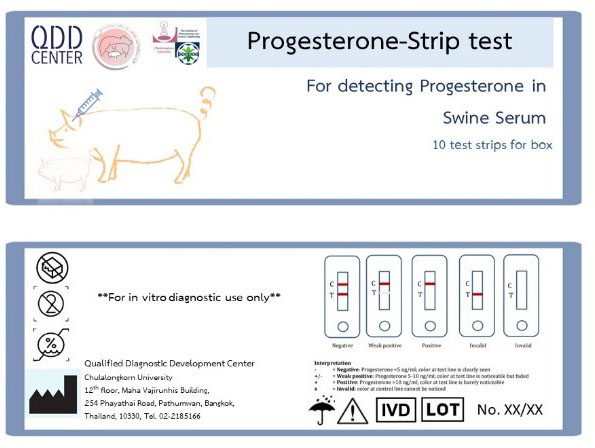
Progesterone-Strip test for detecting Progesterone in Swine serum
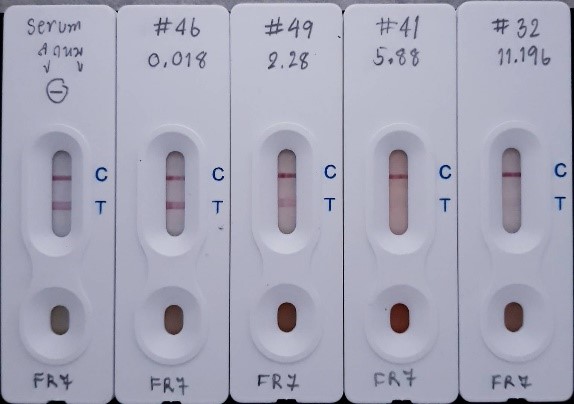
Our paper-based lateral flow test strips offer a convenient means for evaluating reproductive and pregnancy readiness in sows. This kit uses a competitive immunoassay with a highly specific antibody to detect progesterone in sow sera, providing both qualitative and semi-quantitative results that can be easily interpreted within 15 minutes. Positive and negative results indicate progesterone concentrations ≥ and ≤ 10 ng/mL of serum, respectively.
Type: Competitive lateral flow assay
Applications: Qualitative and semi-quantitative detection of progesterone in pig sera. The information obtained can be used to guide the selection of sows for breeding purposes and formulate effective strategies for managing sows that are not yet ready for reproduction.
Dynamic range:
- Positive for ≥ 10 ng progesterone /mL of serum
- Negative for ≤ 10 ng progesterone /mL of serum
Rapid Lateral Flow Immunochromatographic Strip Tests (LFICS) for the Detection of Albumin in Urine
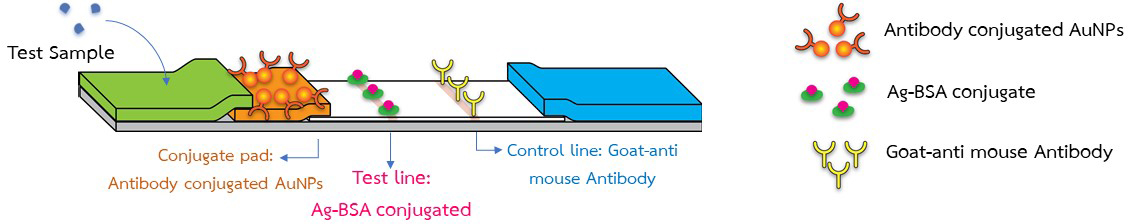
Utilizing a competitive immunoassay with a specific antibody, this rapid lateral flow test kit requires less than 15 minutes to detect as little as 20 µg microalbumin /mL of urine sample
Type: Competitive lateral flow assay
Application: Point-of-care (POC) screening for microalbuminuria, an indicator of early stages of kidney disease.
Dynamic range:
- Positive for ≥ 20 µg microalbumin /mL of urine sample
- Negative for ≤ 20 µg microalbumin /mL of urine sample
Advantages: Highly accurate, lightweight, easily disposable, and field-deployable
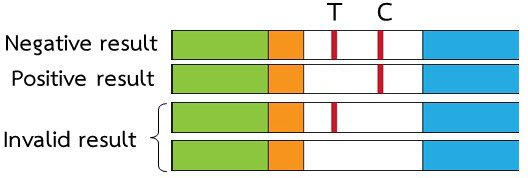
The presence of a strong, reddish purple test line (T) indicates a negative result (≤ 20 µg microalbumin/mL of urine sample). The absence of the test line indicates a positive result (≥ 20 microalbumin/mL of urine sample)
Service
Our services encompass the development of diagnostic test kits, prototype construction, and method validation. All of these activities are conducted in certified facilities equipped with skilled personnel, reliable instruments, and a quality management system for medical devices, in full compliance with international standards for lateral flow-based diagnostics. Therefore, our clients can have full confidence in the accuracy, precision, robustness, and suitability for commercialization of the diagnostic methods and prototypes that successfully pass our rigorous validation.
- Construction of prototypes for lateral flow test strips
- Method Validation
- Compound analysis using HPLC
Our expertise
QDD specializes in research and services related to lateral flow test strips, including assay development, prototype construction, and method validation. We also reach out to researchers in and out of the university and actively facilitate the translation of their laboratory diagnostic methods into commercial kits that are thoroughly validated and comply with various standards.
Equipment and Facilities
The lateral flow equipment platform in QDD includes Dispensing System, Cutting machine, and Continuous sealer
Room
Our laboratory is a Class 100,000 clean room with HVAC-controlled temperature, humidity, and pressure, which meets the standards for lateral flow strip production. We also maintain a high level of microbial cleanliness in accordance with the criteria specified in the Standard Methods for Examination of Water and Wastewater (24th Edition).



Contact
qddcenter@gmail.com
+66 2218 5136, +66 2218 5166
+66 2218 5166
12th floor, Maha Vajirunhis Building, Faculty of Science, Chulalongkorn University

