
Our expertise
ADDC is dedicated to serving as an incubator for the development of diagnostic technologies, particularly in the areas of medical diagnostic and food safety in order to facilitate the translation of research findings into commercial products. Examples of our activity include:
- Supporting the infrastructure for research, develop, manufacture and standardization of a lateral flow test kit for cosmetic and food safety (hydroquinone, acidity, and mercury) (Chemistory Co.,Ltd.)
- Development of quality standard for Covid-19 detection kit based on one-step colorimetric RT-LAMP by Zenostics Co.,Ltd. (approved for EUA by Thailand FDA, number T6400110, and ISO13485 certified)
- Contract research and contract manufacturing for diagnostic platforms such as protein microarray, DNA microarray, RT-LAMP, and RT-PCR (Zenostics Co.,Ltd.)
- Diagnostic technology for tumour-derived Circulating Endothelial Cells (tCECs) that requires minimal blood samples, and blood biopsy for detection of cancer biomarkers (International Diagnostics Co., Ltd.)
History
ADDC was established to support startups or ventures interested in leveraging the technologies, knowledge, or expertise of the university to create innovation with high potential for commercialization, particularly diagnostic kits for medical, food safety, and cosmetics safety purposes. As an incubation center aiming at fostering the development of businesses and technology incubation, we are committed to guiding new startups and ventures through critical early stages and helping them cross the “Death Valley” The support provided by our center encompasses preparing products for certification in accordance to standards such as ISO or GMP, and providing standardized facilities for product development. To achieve these objectives, the following operational strategies will be deployed:
Strategy 1: Actively promote and support startups to acquire certification for their products and production processes in accordance with the relevant standards, including
- ISO 13485 for molecular diagnostic test kits (including RT-LAMP, RT-PCR) and DNA microarray products by Zenostics Co., Ltd.
- ISO 15189 standard (Medical laboratories — Requirements for quality and competence) for liquid biopsy by International Diagnostics Co., Ltd.
- ISO 9001 standard for cosmetic and food safety test kits by Chemistory Co., Ltd. Additionally, in collaboration with Chemistory Co., Ltd., we developed facilities for production of lateral flow diagnostic kits to meet the diverse manufacturing needs.
Strategy 2: Establish a laboratory quality management system to support the incubation of technology and diagnostic methods, ensuring their successful transformation into certified commercial products across a wide range of applications. Additionally, the center is committed to sharing knowledge in product development with interested individuals, as well as offering contract research and contract manufacturing services.
Strategy 3: Foster and actively engage in networking and consultation activities. Our center is an active member of the CEMB network comprising 7 prominent universities across Thailand, as well as the Yothee Medical District project and other collaborative initiatives. These partnerships aim to attract potential collaborators, investors, and technology users, creating an ecosystem for the development and production of diagnostic technologies.
Rationale for Establishing
Mahidol University has received support from Innohub as part of a project that aims to strengthen Thailand's innovation-based economy, in alignment with the Thailand 4.0 policy that seeks to promote the translation of technologies from the laboratory to the industrial/commercial scale. This project is a continued effort to create new startups in 10 Thai S-Curve industries, including the medical hub industry and food processing industry, and to serve as an incubator that supports startups in advancing the technology readiness level (TRL) of their innovation to TRL7-9 (production and practical usage stages). To this end, the project offers standardized facilities and frameworks to ensure that the products and relevant operations meet the stringent quality and safety standards for medical, health, and food products. This initiative is also supported by CEMB, with emphasis on the development of commercial diagnostic kits for medical and food safety purposes based on research outputs from Mahidol University and other network members. Examples of related startups and ventures include:

ZENOSTIC CO., LTD.
Offer contract research and contract manufacturing for communicable and non-communicable diseases for the molecular diagnostic platform including RT-PCR, RT-LAMP, DNA microarray and related reagents using in the process. Our products cover in the field of human, livestock, food processing, in-line monitoring and environmental detection.

CHEMISTORY CO., LTD.
Production of detection kits for prohibited substances in cosmetics and food products. Production of chemical lateral flow kits for dry chemistry applications.

INTERNATIONAL DIAGNOSTICS CO., LTD.
Development of cell bank for the detection of tumour-derived Circulating Endothelial Cells (tCECs) by using low-volume blood specimens
In alignment with our objectives, our center is equipped with the following spaces and facilities:
- Clean room Class 10,000 for production of kits
- Spaces and facilities dedicated to the production of microarrays for applications involving protein and DNA, and the production of molecular diagnostic kits such as RT-PCR and RT-LAMP, etc. Also supported are relevant operations such as process design, quality control tests, and product packaging
- Spaces and facilities for manufacturing of lateral flow devices, as well as their validation, quality control, and packaging
- Cell culture room for culturing cancer cell lines that are used as the positive control in Circulation Endothelial Cells (CEC) of prostrate cancer screening test using magnetic sorting and immunofluorescent. Laboratory for the detection of cancer cells using magnetic sorting and fluorescent dye staining
- Storage facility and space for clinical samples waste management prior to disposal
In addition, the laboratory is equipped to support the development of diagnostic kits for other diseases and hazardous substances, which opens up possibilities for new applications in medical, health, and food safety in the future.
The project provides support in the forms of laboratory space, research equipment, and standardized manufacturing facilities for the development of commercial diagnostic kits. We encourage researchers from Mahidol University and other universities in the network to leverage the resources that we offer in translating their research findings to commercial products (TRL 8-9). The nominal fees charged for space rental will provide users with a realistic understanding of expenses involved in product development, and will be allocated towards the maintenance of facilities and equipment. In line with the Thailand 4.0 policy, the products that this laboratory supports include medical devices, detection kits for applications in the food industry. Currently, our center and participating startups are working towards obtaining certification for the following standards:
- ISO 9001: Quality management system
- ISO 13485: Quality management system for industrial production of medical devices and tools
- ISO 15189 and ISO 15190: Quality and competence in medical and forensic laboratories, safety in medical laboratory
Objectives and scope
- To support the management of the laboratory to ensure seamless operation and provision of services in technology incubation and venture incubation.
- To provide support for startups in a variety of aspects, including consultation, preparation of documents for certification, and assistance in applying for relevant certifications and approvals such as ISO (ISO 9001, ISO 13485, ISO 15189, and ISO 15190), GMP, and FDA Thailand approval.
- To promote the initiation of new businesses, products, services, and jobs in technology-driven economy.
Products and Services with International Standards
- Zenostics Co., Ltd., a startup affiliated with the Advanced Diagnosis Development Center (ADDC), has received certification for ISO9001 (No. FM751175) and ISO13485 (No. MD751174), with the scope “the design, development and manufacture of nucleic acid in-vitro diagnostic test kits for infectious diseases.” The Fastproof 30-min TTR SARS-CoV-2 RT-LAMP Kit has been certified by British Standards Institution (BSI)
- Chemistory Co., Ltd., a startup affiliated with the Advanced Diagnosis Development Center (ADDC), has been certified for ISO 9001:2015, with the scope “Design, Development and Manufacturing of Test Kits for Cosmetic and Food Safety”
- International Diagnostics Co., Ltd. (also known as X-Zell Biotech Co., Ltd.), a startup affiliated with the Advanced Diagnosis Development Center (ADDC), has been certified for ISO 15189:2012 and ISO 15190:2020 (medical laboratories)
Products and Innovation
FastProof™ 30 min-TTR SARS-CoV-2 RT-LAMP Kit
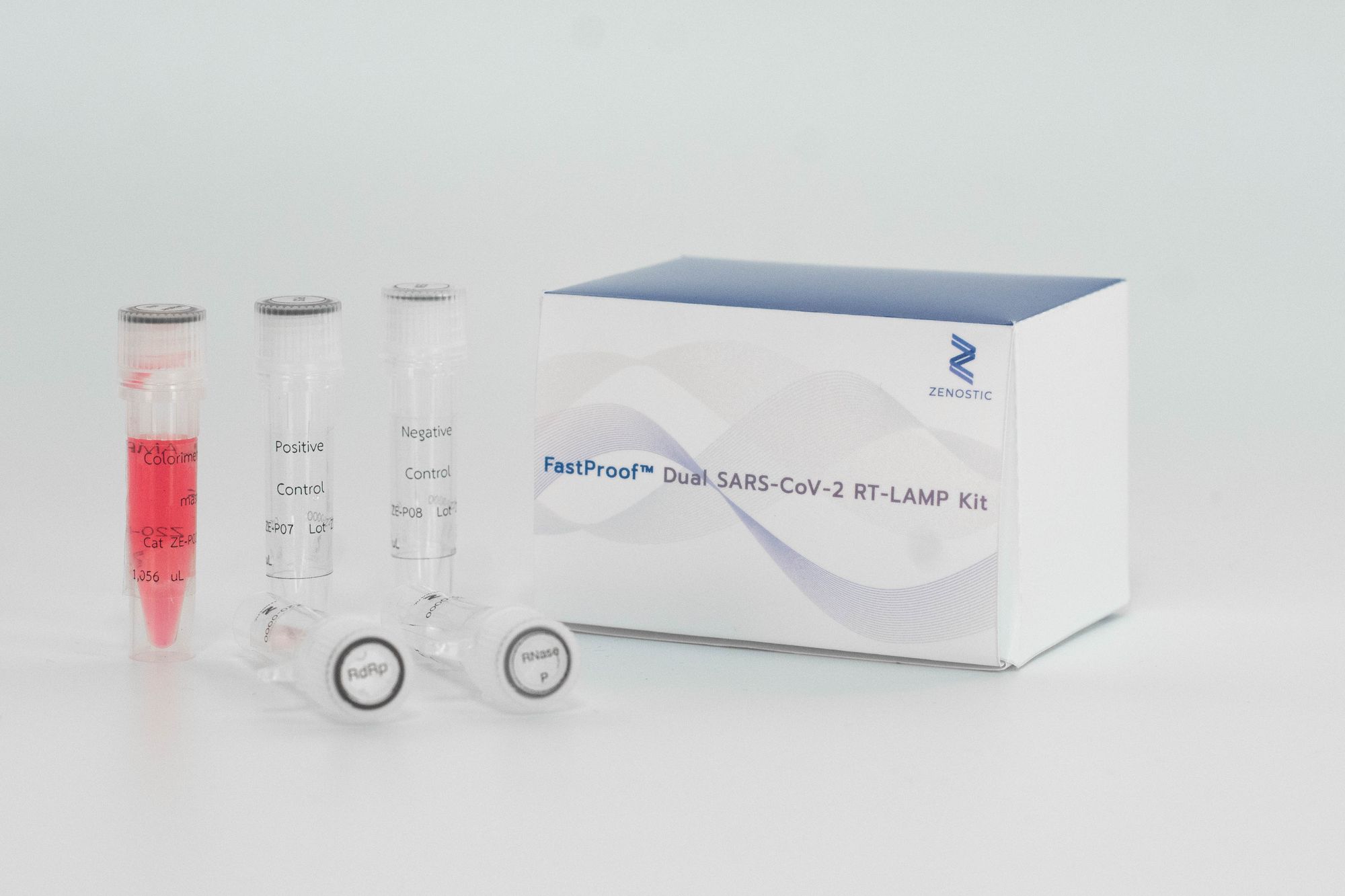

Description
The FastProof™ 30 min-TTR SARS-CoV-2 RT-LAMP Kit employs the principle of colorimetric RT-LAMP to detect RNA from SARS-CoV-2 in samples obtained from throat or nasopharyngeal swabs. The amplification of target nucleic acids results in a noticeable color change in the solution, indicating the presence of SARS-CoV-2 genetic material.
Intended use
RT-LAMP is an alternative method of RT-PCR for SARS-CoV-2 detection where the samples from nasal and throat swab can be diagnosed.
Indication
This product is a rapid diagnostic kit for SARS-CoV-2 that provides qualitative results. The test is based on the colorimetric RT-LAMP method, which enables naked-eye visualization. It is designed as an in vitro diagnostic kit exclusively for use by medical professionals.
- Package: 1 kit (box) contains 60 reactions
- Each kit contains:
- 1 tube (1,200 microliters) of Color LAMP-TTR (1,200 microliters)
- 2 tubes (300 microliters each) of Primer Mix, each containing primers mix-TTR and RNase P primers mix-TTR (internal control)
- 1 tube (60 microliters) of Positive control-TTR
- 1 tube (60 microliters) of Negative control-TTR (RNase-free water)
Related standards
- ISO 9001:2015 Quality Management Systems
- ISO 13485:2016 Medical devices - Quality Management Systems
Research and Produced by ZENOSTIC CO., LTD.
Test kits for cosmetic and food safety

Four different types of kits are available and can be purchased separately. These kits demonstrate higher efficiency than other commercial kits in the market.
Related standards
ISO 9001:2015
*Mercury Test Kit has been certified by the National Institute of Metrology (Thailand)
Research and Produced by CHEMISTORY CO., LTD.
Mercury test
Hydroquinone test
Acidity test
Formalin test kit for food
Test kit for prostate cancer cells in blood circulation
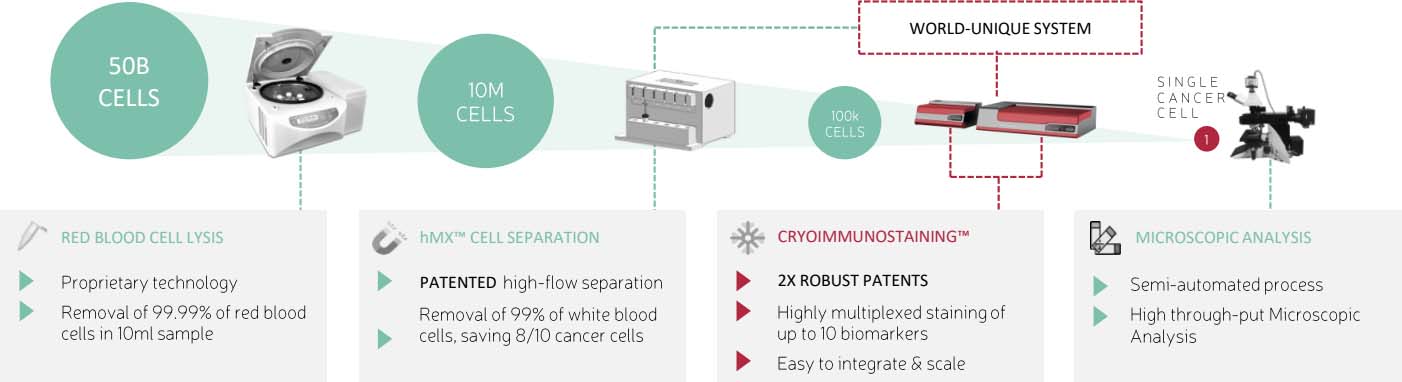
The measurement of prostate specific antigens (PSA) in blood circulation has been commonly utilize for prostate cancer screening. However, this approach is limited in the ability to distinguish between prostate cancer and other prostate conditions, and therefore must be supplemented by prostate biopsy. However, since biopsy is considered to be an invasive procedure, it can cause stress and uneasiness for the patient. Therefore, as a way to eliminate the stress and uneasiness for the patient, International Diagnostics Co., Ltd. (also known as X-zell Technology) initiated the liquid biopsy service for detecting prostate cancer cells in blood circulation. The target cells will then be stained with 8 different fluorescent antibodies in order to enhanced accuracy and aid in the diagnostic decision-making process. Due to its efficiency and minimal invasiveness, this approach offers an attractive option for individuals seeking initial cancer screening without any risks of injury and pain.

The liquid biopsy prostate cancer test kit detects CEC/CTC (Circulating Endothelial Cell/Circulating Tumor Cell.) CEC are endothelial cells that line the blood vessels required for the cancer cell to grow. CEC can be detected in the blood vessel at an early stage of prostate cancer. The kit utilize biomarker in order to detect these CEC. On the other hand, CTC cells are cells that migrate from the primary tumor into the blood circulating, which are able to use as the prostate cell biomarker in later stage.
The key advantage of the detection kit developed by International Diagnostics Co., Ltd. is its suitability for early cancer screening by using of blood samples that will reduce the risk of side effects, reduce pain, and minimizing the recuperation period in hospitalization when comparing to patient who have to perform prostate biopsy. However, it is important to note that this test is one of the options for preliminary screening of abnormal cells — in certain cases, patients may still be required to consult a physician to determine whether this test is suitable for their situations.
Furthermore, the company produce and supply reagents for diagnostic kits. This operation has been certified with the ISO 13485:2016 standard for medical devices.
To ensure the quality of services provided by our laboratory, the company has received certifications for ISO 15189:2012 and ISO 15190:2020, which focus on the quality and competence in medical laboratories and safety in medical laboratories, granted by the Bureau of Laboratory Quality Standards under the Department of Medical Sciences, Ministry of Public Health. These initiatives have been financially supported by the Advanced Diagnosis Development Center (ADDC)
Research and Produced by INTERNATIONAL DIAGNOSTICS CO., LTD.
Service
CHEMISTORY CO., LTD.
Detection of mercury in cosmetics. Please contact us for pricing and additional details (Tel: +66 2201 5848).
INTERNATIONAL DIAGNOSTICS CO., LTD.
Detection of circulating prostate cancer cells using liquid biopsy. Please contact us for pricing and additional details (Tel: +66 2087 8499)
Our expertise
- Research, development, and manufacturing of lateral flow devices (Chemistory Co., Ltd.)
- Test kits for food safety (Chemistory Co.,Ltd.)
- Test kits for cosmetics safety (hydroquinone, acidity, and mercury) (Chemistory Co.,Ltd.)
- One-step colorimetric RT-LAMP kit for rapid COVID-19 detection by Zenostics Co., Ltd. (Approved for EUA by FDA Thailand, Number T6400110; ISO13485 certified)
- Contract research and contract manufacturing in areas related to microarray technologies (Zenostics Co., Ltd.)
- Detection technology for tumour-derived Circulating Endothelial Cells (tCECs) requiring minimal volumes of blood samples
Equipment and Facilities
- Biological Safety Cabinet (BSC) Class II A2
- Microarray Spotter
- XYZ3210 Dispense Platform
- CM5000 Guillotine Cutter
- X-ray fluorescence spectrometer (XRF, S2 PUMA Single)
- CO2 Incubator
- Inverted Microscope
Room
- Clean room Class 10,000 for manufacturing purposes
- Spaces for production of microarrays for applications involving protein and DNA, and molecular detection kits such as RT-PCR and RT-LAMP, among others. The spaces provided can also accommodate other operations such as process design, quality control, and packaging
- Space for manufacturing, testing, quality control, and packaging of lateral flow devices
- Laboratory for detection of cancer cells using magnetic sorting and fluorescent dye staining
- Room for storage and destruction of clinical samples prior to disposal



Contact
+66 2201 5842
+66 2201 5843
Room K 604, 6th Floor, Chalermprakiat Building Faculty of Science, Mahidol University
272 Rama 6 Road Ratchatewi, Bangkok 10400
CHEMISTORY CO., LTD.
+66 2201 5848https://www.facebook.com/MercUryTestKit/
ZENOSTIC CO., LTD.
+66 2201 7244https://www.facebook.com/Zenostic/
info@zenosticbio.com
zenosticbio.com
INTERNATIONAL DIAGNOSTICS CO., LTD.
+66 2087 8499simaporn.p@x-zell.com
www.x-zell.com
241/1 Soi. Phiboon Wattana Buildings, Rama 6 Road, Phayathai, Bangkok 10400

