


Our expertise
Our center specializes in development of diagnostics and medical devices, such as whole blood quality control materials and urine quality control materials. We are equipped with the following certified facilities:
- Humidity-controlled laboratory
- Clean room
- Medical device manufacturing establishment that has been registered with FDA Thailand, for In Vitro Diagnostic (IVD) medical devices including blood collection tubes, quality-control materials
History / Rationale for Establishing
The Medical Device Research Laboratory at Naresuan University was founded as part of an endeavor to renovate the laboratory facilities within the university into dedicated space for translating research outputs into medical device prototypes according to standards such as GMP or ISO 13485, as well as relevant regulations. Operated under the MOU between Mahidol University and Naresuan University, the center also functions as a medical laboratory that conforms to the ISO 15189 and 17025 standards, equipped to accommodate translational research conducted within Naresuan University and the collaboration network. Initially, the laboratory will focus on producing the prototype of blood collection tubes which coated with an anticoagulant developed from the research of Naresuan University.

In the first phase (2019-2020) of renovating the Medical Device Research Laboratory and producing prototype blood collection tubes that meet the ISO 13485 standard, we will use the facility and equipment provided by the project "Innovation Hub - Aging Society project to create an innovation-based economy of the country according to the Thailand 4.0 policy and to support the development of infrastructure for the development of diagnostic products under the special anticoagulant-coated blood collection tube product project, medical device standard for small blood volume collection tube for laboratory diagnosis in the elderly group" This project, led by Associated Professor Sarawut Kumphune, has received funding of 25,710,000 baht and spans 3 years, from December 12, 2017 to December 11, 2020. The development of prototype blood collection tubes is supported by the project “Development of production process and efficiency evaluations of blood collecting tube for elderly people” which has received funding of 18,312,000 bath and is led by Associated Professor Wanvisa Treebuphachatsakul, for a duration of 1 year from October 1, 2018 to September 30, 2019.
Objectives and scope
- To enhance the available laboratory facilities to meet the standards required for manufacturing medical device prototypes.
- To establish a medical laboratory dedicated to research activities in medical sciences and construction of medical devices.
- To establish Naresuan University as a hub for disseminating knowledge in medical technologies and innovations, systems for manufacturing of medical devices, relevant regulations, and laboratory standards to researchers, business owners, and students.
Products and Services with International Standards
- Our quality management system for medical devices has been certified in accordance with ISO 13485:2016 & EN ISO 13485:2016, with the scope encompassing “the manufacture of substance glucose, hematocrit, hemoglobin A1c, substances in urine for in vitro diagnostic tests and blood collection tube for clinical laboratory testing.”
- The standard for quality and competence in medical laboratories (ISO 15189:2012)
- The standard for safety in medical laboratories (ISO 15190:2020)
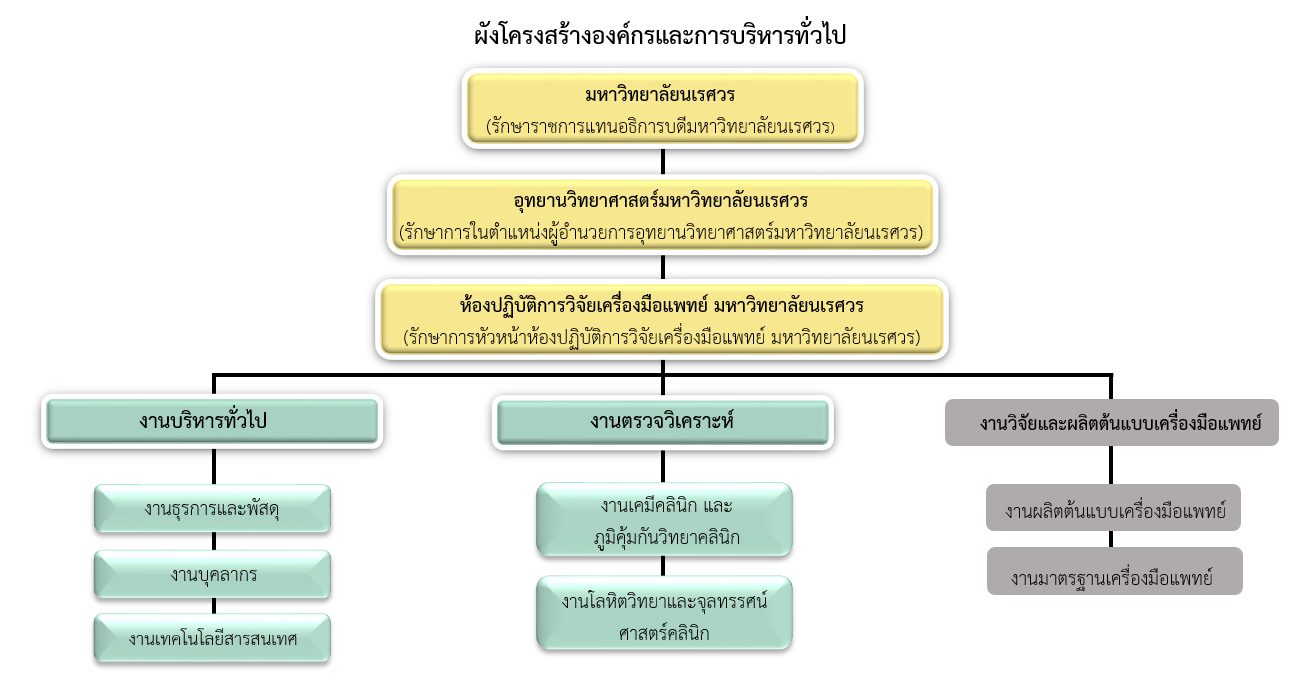
Organizational chart
Products and Innovation
Innomed blood collection tubes
Our lithium heparin blood collection tubes contain inhibitors of in vitro glycolysis and allow for the measurement 19 biochemicals, including Glucose, Cholesterol, Triglyceride, HDL, LDL, BUN, Creatinine, Uric acid, Na+, K+, Cl-, HCO-3, AST, ALT, ALP, Total bilirubin, Direct bilirubin, Albumin and Total protein. By using Innomed tubes, blood sugar levels can be preserved for up to 8 hours without compromising the testing of other biochemicals. While two different types of conventional tubes are required to measure both glucose and other biochemicals — a lithium heparin tube for biochemical measurements and a sodium fluoride tube for glucose measurement — a single Innomed tube can serve both purposes. This versatility decreases plastic usage in laboratories and minimizes the amounts of blood required per patient.
How to use
- Inspect the tube for any defects or abnormalities
- Wear personal protective equipment (PPE)
- Follow the standard procedure for venipuncture (i.e., collection of blood from veins) in diagnostic laboratories
- The vacuum inside the tube creates suction that will draw blood into the tube until it reaches the indicated fill level
- Immediately mix the collected blood with anticoagulant by gently inverting the tube
- With an Innomed 02 tube, blood components can be separated immediately after venipuncture. This can be achieved by centrifuging the tube at 3,500 RPM for 5-10 minutes. In the case of an Innomed B tube, centrifugation should be performed 15 minutes after blood mixing (Step 5)
- Place the tube on an automated blood analysis device and follow the steps outlined in the standard operating procedures (SOPs) of the laboratory
Benefits of the product
- Using Innomed tubes reduces the amount of blood collected per patient by 33-50 percent and eliminates the need for repeated punctures, thereby enhancing the convenience and efficiency of blood collection services
- Blood sugar levels can be preserved for up to 8 hours, significantly longer than conventional lithium heparin tubes that allow for only 2 hours of blood sugar preservation. This extended preservation time prevents underestimation of blood sugar levels and increases the credibility of blood measurement reports.
- Blood collected in a single Innomed tube can be used for the analysis of both blood sugar and other biochemicals, reducing the number of tubes required per patient from 2 to 1.
- Decreased tube usage has the potential to reduce hospital expenses by 33-50 percent as well as minimize the amount of biohazardous wastes generated
- This innovation is developed and manufactured in Thailand, and has received certification according to international standards



Q-prompt quality-control material
Q-prompt is a quality-control material based on whole blood, prepared by chemically fixing red blood cells and incorporating additives to preserve blood cells and eliminate microbes. This process can be used to prepare whole blood samples using surplus donated blood from blood banks, instead of collecting fresh blood from volunteers or patients, making it highly suitable for commercial-scale production. In addition, the whole blood used in the production of Q-prompt is of high quality, characterized by stable sugar levels and absence of hemolysis. The product can be transported by mail without the need for cold storage. Therefore, Q-prompt offers a high-performance, cost-effective, and domestically produced control material that rivals imported alternatives. Versatile applications of Q-prompt include standardization of portable blood sugar detectors and measurement of hematocrit in urban and rural hospitals.
Service
| NO. | Sample | Test | Test method |
|---|---|---|---|
| 1. | Serum/Heparin, EDTA Plasma | Albumin | Colorimetric method/ Bromocresol green (BCG): Cobas c111 Analyzer |
| 2. | Serum/Heparin Plasma | Alkaline Phosphatase (ALP) | Colorimetric method/ Bessey-lowry-brock): Cobas c111 Analyzer |
| 3. | Serum/Heparin, EDTA Plasma | Alanine Aminotransferase (ALT) | Enzyme kinetic method): Cobas c111 Analyzer |
| 4. | Serum/Heparin, EDTA Plasma | Aspartate Aminotransferase (AST) | Enzyme kinetic method): Cobas c111 Analyzer |
| 5. | Serum/Heparin, EDTA Plasma | Direct bilirubin | Colorimetric method/ modified jendrassik and grof: Cobas c111 Analyzer |
| 6. | Serum/Heparin, EDTA Plasma | Total bilirubin | Colorimetric method/ modified jendrassik and grof: Cobas c111 Analyzer |
| 7. | Serum/Heparin, EDTA Plasma | Blood Urea Nitrogen (BUN) | Enzyme kinetic method/ urease-glutamate dehydrogenes : Cobas c111 Analyzer |
| 8. | Serum, NaF Plasma | Glucose | Enzymatic colorimetric method: Cobas c111 Analyzer |
| 9. | Fresh capillary/ Heparin,EDTA whole blood | Glucose | Blood glucose meter |
| 10. | Serum/Heparin Plasma | Cholesterol | Enzymatic colorimetric method/cholesterol oxidase-peroxidase: Cobas c111 Analyzer |
| 11. | Serum/Heparin, EDTA Plasma | Creatinine | Enzymatic colorimetric method: sarcosine oxidase Cobas c111 Analyzer |
| 12. | Serum/Heparin, EDTA Plasma | HDL-Cholesterol | Enzymatic colorimetric method: Cobas c111 Analyzer |
| 13. | Serum/Heparin, EDTA Plasma | LDL-Cholesterol | Enzymatic colorimetric method: Cobas c111 Analyzer |
| 14. | Serum/Heparin, EDTA Plasma | Triglyceride | Enzymatic colorimetric method /glycerokinase peroxidase-peroxidase: Cobas c111 Analyzer |
| 15. | Serum/Heparin, EDTA Plasma | Total Protein | Colorimetric method/ biuret method: Cobas c111 Analyzer |
| 16. | Serum/Heparin, EDTA Plasma | Uric acid | Enzymatic colorimetric method /uricase-peroxidase |
| 17. | Serum/Heparin Plasma | Lactate dehydrogenase (LDH) | Colorimetric method: Cobas c111 Analyzer |
| 18. | EDTA, Heparin, Oxalate whole blood | Hemoglobin A1c | Turbidimetric inhibition immunoassay: Cobas c111 Analyzer |
| 19. | EDTA whole blood | Complete Blood Count (CBC) | -Electrical impedance -Spectrophotometry -Numeric Integration: Pentra 80XLR Analyzer |
| 20. | EDTA whole blood | Blood smear | Centrifugation |
| 21. | Fresh capillary, EDTA whole blood | Hematocrit | ion-selective electrode potentiometry: i-STAT analyzer |
| 22. | Heparin whole blood | Sodium (Na+) | ion-selective electrode potentiometry: i-STAT analyzer |
| 23. | Heparin whole blood | pH | ion-selective electrode potentiometry: i-STAT analyzer |
| 24. | Heparin whole blood | pO2 | ion-selective electrode potentiometry: i-STAT analyzer |
| 25. | Heparin whole blood | pCO2 | ion-selective electrode potentiometry: i-STAT analyzer |
| 26. | Heparin whole blood | HCO3 | Henderson-Hasselbalch: i-STAT analyzer |
| 27. | Heparin whole blood | Potassium (K+) | ion-selective electrode potentiometry: i-STAT analyzer |
| 28. | Heparin whole blood | Chloride (Cl-) | ion-selective electrode potentiometry: i-STAT analyzer |
| 29. | Heparin whole blood | Ionized Calcium (Ca2+) | ion-selective electrode potentiometry: i-STAT analyzer |
| 30. | Urine | Urine strip test | Reflectance photometer (Dual Wavelength), URIT-50 Analyzer |
| 31. | Urine | Human chorionic gonadotropin (hCG) | Immunochromatographic assay |
| 32. | Serum/ Heparin, EDTA, NaF/K oxalate Plasma | Thyroid stimulating hormone (TSH) | ECLIA (electrochemiluminescence immunoassay): Cobas e411 Analyzer |
| 33. | Serum/Heparin, EDTA Plasma | Free Triiodothyronine (FT3) | ECLIA (electrochemiluminescence immunoassay): Cobas e411 Analyzer |
| 34. | Serum/Heparin, EDTA Plasma | Free Thyroxine (FT4) | ECLIA (electrochemiluminescence immunoassay): Cobas e411 Analyzer |
| 35. | Serum/Heparin, EDTA Plasma | Carcinoembryonic Antigen (CEA) | ECLIA (electrochemiluminescence immunoassay): Cobas e411 Analyzer |
| 36. | Serum/Heparin, EDTA Plasma | Total Prostate-Specific Antigen (PSA) | ECLIA (electrochemiluminescence immunoassay): Cobas e411 Analyzer |
- Central laboratory
- Clean room
- Humidity-controlled room
Our expertise
- Whole blood quality-control material
- Urine quality control material
Equipment and Facilities
- Biosafety Cabinet Class ll (BSC)
- Clean Booth
- Incubator
- Accelerated Weathering Tester
- CO2 Incubator
- Refrigerate Shaking Incubator
- Freeze Dryer
- Fume Hood
Room
- Clean Room Class 100,000
- The Laboratory accredited by ISO 15189:2012 standard for quality and competence in medical laboratories
- The Laboratory accredited by ISO 15190:2020 standard for safety in medical laboratories



Contact
+66 5596 8772, +669 8525 9414
Medical Device Research Laboratory at Naresuan University
Mahathammaraja Building Zone C, First Floor, Room TC104 - TC018

